Investors
Quality Management
What Quality means to Fresenius Kabi Oncology Limited
For Fresenius Kabi Oncology Limited, commitment towards improving the quality of life in a patient is imperative. The Company commits towards an effective Quality Management procedure in terms of assuring higher safety and efficacy in the quality of our products. Our procedures follow the cGMPs and all the necessary certifications and guidelines to maintain full compliance with the Pharmaceutical RegulatoryAuthorities thus meeting the expectations of our partners and customers. For us, it is an ongoing task to undertake continuous quality improvements across all operations with the aim to support medical professionals in the best-possible therapy and care for critically and chronically ill patients around the globe.
We, at Fresenius Kabi Oncology Limited, try to ensure that every individual accepts continual improvement of its products, processes and systems as an objective. Also, effective decision making is an important aspect of Fresenius Kabi Oncology Limited which is based on the analysis of data and information.
The basis for our lasting and successful partnership with our customers is the consistently high and ever improved quality of our products and services. Therefore, our quality management system according to the international ISO 9001 standard is regularly reviewed during internal quality audits and certified by external auditors.
Good documentation is an essential part of the QM System and is pre-requisite for compliant operations. Structure of Quality Management Documents at Fresenius Kabi is explained below:
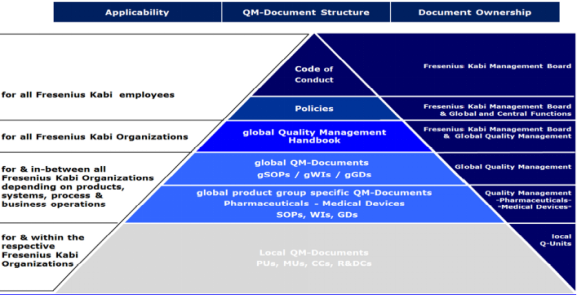
Quality Policy Fresenius Kabi Oncology Limited
Our products and our services, as well as the commitment and dedication of our employees, are focused on the three aspects: Quality, Safety and Efficacy.
The Quality Management System (QMS) is designed to increase transparency, improve internal processes and allow the company to become more effective. We are striving for worldwide harmonized standards and processes to guarantee highest quality profiles.
Achieving cost leadership by supplying sufficient quantities in world class quality and the development and roll out of innovative products and technologies is one key element of a sustainable and profitable growth of Fresenius Kabi Oncology Limited.
Fresenius Kabi Oncology Limited completely understands the value of life; hence, it ensures that drugs, medicines and injections are completely safe and effective when people consume them. Since it is a matter of health and life, Fresenius Kabi Oncology Limited does not compromise on the quality standards of the various drugs and other health related products that it develops and manufactures. National and international authorities constantly check and monitor whether the manufacturers are strictly adhering to quality regulations. The Company has a robust supplier qualification process in place to have control on quality of input materials. This helps the company follow quality requirements and meet standards in development of its products as well as in maintaining mixers and packing lines.
Continuous improvement is an essential element in a modern quality system. Many new technologies are currently available to the company that provides information on the physical and chemical entities to improve process understanding and to measure, control, and to have a proactive approach and predict quality and performance. The guidance facilitates the introduction of such new technologies to improve efficiency and effectiveness of manufacturing process design and control and quality assurance.
Manufacturing based on Innovation and Continuous Improvement in Pharmaceutical Product Development has led to the following achievements by the company.
- Facilitating continuous processing to improve efficiency and better management
- Developing additional guidance on quality systems for pharmaceutical manufacturing to enhance and modernize pharmaceutical manufacturing and product quality
- Correct implementation of cGMPs and meeting internal and international goals
- Continuous effective communication and compliance with FDA requirements


 Send link to a friend
Send link to a friend